There are always warnings about gas dryers producing carbon monoxide, but there is very little data on exactly how much they produce. Without evidence from clinical research and studies, we can only puzzle it out using what we do know.
While there is not much in terms of statistics for gas dryers’ carbon monoxide production, we can use logic to make educated guesses about how great a risk this poses. This is, however, just logical deductions using the available information and may not be a wholly accurate representation.

There is no clear answer for how much CO a gas dryer produces. The safe exposure for CO is 0.01% of that of CO2. Using this, it is possible to estimate a possible safe level per cycle. However, the exact composition of combustion gases from incomplete combustion can differ.
Negligible Amount if the Dryer Is Working Properly
The amount of carbon monoxide (CO) produced by a gas dryer should have no significance. A gas dryer that is correctly installed and functioning optimally should produce little to no CO since it ideally produces carbon dioxide (CO2) as the primary byproduct.
Combustion gases are the byproduct of the combustion process. Combustion is a chemical reaction that takes place between oxygen (from the combustion air) and the fuel for the dryer. This process adds oxygen to the fuel to produce heat (exothermic reaction).
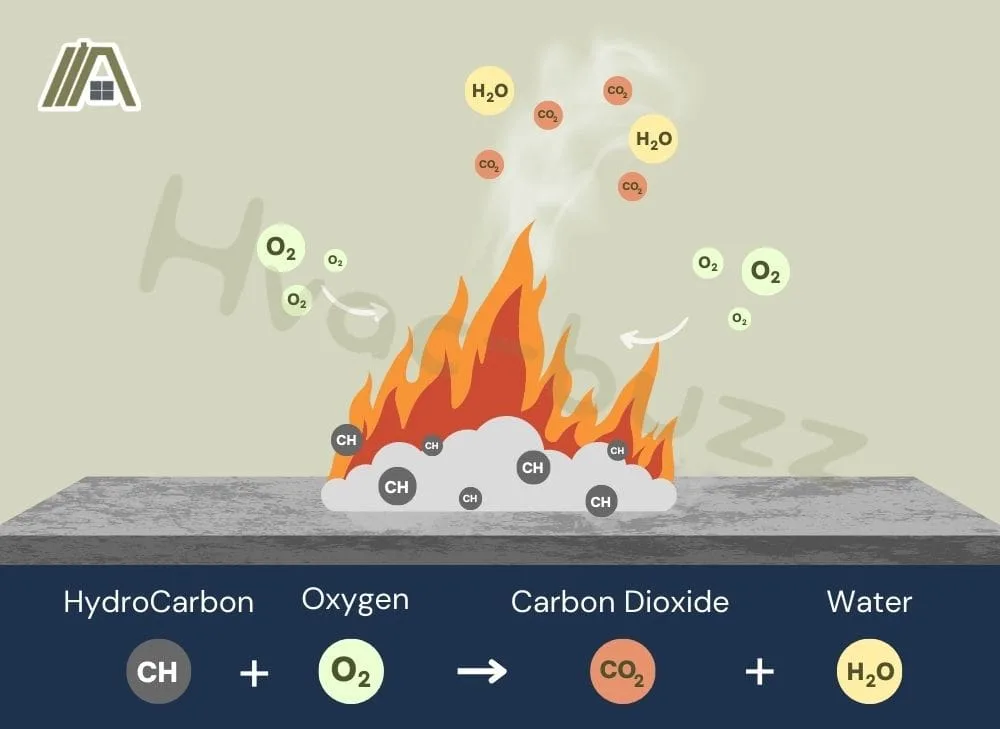
The fuel contains hydrocarbons. During combustion, oxygen is added to these particles, creating CO2 (and water).
When heat and CO2 are produced, we call this complete combustion.
In a perfect world, there would only be CO2, but with complete combustion small traces of CO are likely to be present, just not enough to harm you.
When the Oxygen Supply is Insufficient
There is always the potential for CO to be produced by the dryer, which is why it is essential to vent the appliance properly.
This potential comes from the fact that insufficient combustion air would mean that there is not enough oxygen for combustion.
During this incomplete combustion, the exothermic chemical reaction will still occur, but less oxygen is added to the carbon molecules creating some CO and less heat.
Additionally, some carbon molecules remain unattached from other elements (not enough oxygen). This appears in the form of soot that you can see gathering in the burner housing.
So, now that we understand how dryers produce CO, let’s work out if the amount produced with incomplete combustion is enough to be concerning.
How Much Carbon Dioxide Do Dryers Produce?
A dryer is likely to produce around 1.8 kg of CO2 on average during a normal cycle if complete combustion occurs.
Something to note is that liquid propane is regarded as more environmentally friendly, which is why it is the choice of many who are concerned with their laundry day’s carbon footprint.
While I cannot find exact figures, it is generally considered to produce 50-60% less CO2, CO, and nitrogen oxide than gasoline because it is a low-carbon source of energy.
So, if you use a propane-fuelled dryer, the amount of CO2 you are dealing with is likely smaller than the average.

Natural gas fuels produce a lot of CO2 even though it is less than fossil fuels. Unfortunately, I could not find statistics on how much is produced or even a percentage comparison to other gas fuels.
Not All Carbon Becomes Carbon Monoxide
So, dryers generally produce around 1.8 kg of CO2. However, before you start doing a direct translation to the amount of CO, you need to be aware of these two factors:
- Some carbon dioxide is still produced during the incomplete combustion. There is simply not enough oxygen to provide two molecules to all the carbon molecules.
- Some carbon remains as elemental carbon since there is insufficient oxygen to react with all the carbon molecules.
Thus, the amount of CO released by a dryer during incomplete combustion is far less than the amount of CO2 it typically creates with a sufficient oxygen supply.
When Does Carbon Monoxide Become Dangerous?
The average dryer cycle produces 3.96 lbs (1.8 kg) of CO2. According to OSHA (2020), the amount of CO2 that you can be safely exposed to must be below 5,000 ppm per 8 hours. This equates to 625 ppm per 1 hour.
The average dryer cycle lasts approximately 30 minutes, making the exposure limit of CO2 312.5 ppm for 30 minutes (per cycle).
The exposure limit for CO is significantly less at 50 ppm per 8 hours, according to OSHA (2012) regulations. This equates to 6.5 ppm per 1 hour and 3.125 ppm per 30-minute cycle.
This means that the safe concentration of CO is less than 0.01% of that of CO2. So, for a dryer to be considered safe in terms of CO production, it needs to produce less than 0.01% of the amount of CO2 produced, or it is toxic.
Of the 3.96 lbs (1.8 kg) of CO2, a maximum of 0.0396 lbs (0.018 kg) of CO is the probable amount that can be produced.
Is the Risk Significant?
Manufacturers will not make dryers that release toxic amounts of CO2, so emissions must be within safe limits. So, the 3.96 lbs (1.8 kg) of CO2 produced will have to fall below the threshold of 312.5 ppm per cycle (the toxic limit).

When estimating the risk from a dryer, we know that the amount of fuel remains the same when burning completely or incompletely, and we know how much CO2 that produces.
We also know that the amount of CO2 produced (3.96 lbs/1.8 kg) must fall under 312.5 ppm to prevent it from becoming toxic at the exposure limit.
We don’t know how much less than that limit is 3.96 lbs, but we can agree that this quantity of CO2 produced over the course of the cycle is safe, or the manufacturers could not sell that appliance.
We can also assume the 0.01% equivalent (0.036 lbs/0.018 kg) of CO is probably under the exposure limit and safe. However, we don’t know how much of the 3.96 lbs (1.8 kg) is in the form of carbon, how much is CO2, and how much is CO when there is incomplete combustion.
However, the likelihood of it falling below the exposure limit is small. If only 10% of the 3.96 lbs of carbon-based byproducts released are released as CO, then this is 0.039 lbs of CO, which is greater than the estimated safe amount of CO (0.036 lbs).
The problem also comes in when you consider other variables that can increase the concentration or presence of CO in the space:
- Other gas-burning appliances can also produce CO.
- Smaller areas mean there is less dilution of CO and possibly more incomplete combustion.
- Closed windows/doors mean there is less air exchange to dilute the CO concentration.
- Exhaust systems can pull combustion gases from the appliance or take combustion air causing incomplete combustion.
- Using the dryer several times a day can cause CO to build to dangerous levels in a room.
- We don’t know how much leeway the manufacturers gave themselves and if that figure is based on a correctly vented dryer, which would disperse the CO in a much bigger area.
Measure Carbon Monoxide Production by Your Dryer
If you are after an accurate CO reading for your specific dryer and room conditions, you can always do some field research.
You can take measurements of the CO levels coming from the dryer using a carbon monoxide meter, like the Lunarlipes Carbon Monoxide Meter (amazon link) or the Fieldpiece SCM4 Carbon Monoxide Detector (amazon link).
This device can provide more accurate, real-time readings for your make and model of dryer. Most of these meters should also have a function that presents the maximum and average measurements that it has taken while powered on.
To operate such a device, you need to turn it on near the appliance or where you are trying to measure the CO levels. This can be in the same room as the dryer or where the dryer exhaust is being emitted, which should be at the vent terminal since combustion gases travel through the drum.
Check the user manual for the device, as some require you to turn it on in a CO-free area to calibrate the reading.
The CO levels shouldn’t exceed 3.125 ppm for a 30-minute drying cycle. You can also monitor if the CO disperses appropriately after the cycle and doesn’t build up.
You also have the opportunity to test the CO levels under different circumstances (such as with windows/doors open or with an exhaust system or gas appliance on) to ensure they stay under the safe exposure limit.
Sources
https://www.chem.fsu.edu/chemlab/chm1020c/Lecture%207/01.php
https://www.theguardian.com/environment/ethicallivingblog/2008/may/02/treadlightlyswitchofftumbl
https://www.diversifiedenergy.com/news/how-does-propane-gas-affect-the-environment/
https://propane.com/environment/stories/propane-made-hydrogen/
https://www.ecoredux.com/is-propane-natural-gas
https://www.homebiogas.com/blog/natural-gas-alternatives/
https://www.griffithenergyservices.com/articles/5-differences-natural-gas-propane
https://www.indsci.com/en/blog/carbon-monoxide-vs.-carbon-dioxide-lets-compare
https://htcinstrument.com/wp-content/uploads/2021/06/CO-01.pdf

